U.S. Supply Chain Security
KEY TAKEAWAYS
- The operations and supply chains of American businesses have been disrupted by measures taken to contain the coronavirus.
- Some lawmakers have questioned U.S. dependence on other countries, such as China, for pharmaceutical ingredients, medical equipment, and other critical products.
- Congress enacted policies to address these concerns in the CARES Act, including ways to track and monitor medical device shortages and increase access to information about the source of pharmaceutical components. Some lawmakers have additional ideas to incentivize companies to move production to the U.S.
The global coronavirus outbreak has disrupted supply chains for U.S. businesses. It has put a spotlight on how heavily the U.S. depends on other countries, such as China, for everything from small electronics to pharmaceutical ingredients. The Coronavirus Aid, Relief, and Economic Security Act included several provisions to try to address shortages of certain medical devices and active pharmaceutical ingredients. Lawmakers have offered other proposals to address supply chain vulnerabilities.
Coronavirus Events Affecting Supply Chains
CORONAVIRUS supply chain disruptions and risks
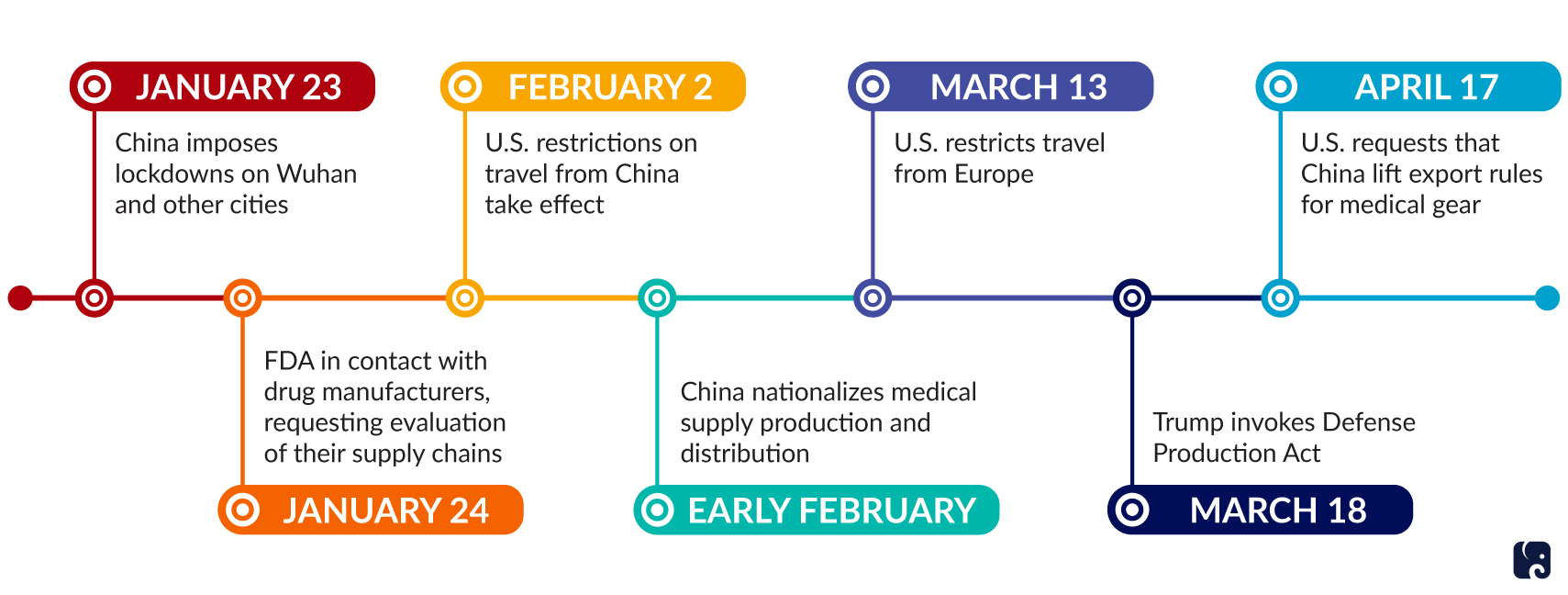
In January, China extended the Lunar New Year holiday to slow the coronavirus’s spread. At least two dozen provinces, city governments, and regions closed businesses until mid-February. China locked down Wuhan, a manufacturing hub, and other cities. The containment measures limited factory production and logistics networks for transporting goods. China’s exports for January and February were down 17% compared to the same two months last year.
While firms in some areas of China have been restarting operations since February, and the lockdown in Wuhan ended April 8, the return to full production has been gradual and uneven. Officials had to remove roadblocks to allow trucks to bring goods to ports. Businesses must follow new protocols, such as providing masks and doing regular temperature checks, and they have faced worker shortages amid quarantines and concerned employees. As a result, American businesses that rely on China for goods such as microelectronics, toys, and clothing have had supply chain disruptions. The Congressional Research Service noted that shortages may continue for several months as shipments from China catch up.
There also have been disruptions to the U.S. medical supply chain. While the U.S. is a net exporter of medical devices, according to the FDA, 13% of the facilities that make active pharmaceutical ingredients are in China. Generic and over-the-counter drugs are especially reliant on foreign supply chains. As of March 21, 54 countries including the United States had imposed trade restrictions on medical supplies to boost their domestic supplies of the goods. In early February, China nationalized medical supply production, determining where supplies go. On February 27, the Food and Drug Administration announced the first U.S. drug shortage associated with the coronavirus outbreak. The agency has contacted drug manufacturers to ensure they notify the FDA of any supply chain disruptions, and it asked manufacturers to evaluate their supply chains. The FDA has taken the same steps with firms that make medical devices and personal protective equipment.
In response, and in an attempt to mitigate future shortages, President Trump signed an executive order on March 18 to invoke the Defense Production Act. He used his authority to ensure the nation has the critical supplies to address the coronavirus outbreak. Private companies such as 3M and General Motors have prioritized the production and distribution of medical supplies. The FDA has issued “emergency use authorizations” for diagnostic tests, ventilators, masks, and other medical devices in an attempt to help speed the production of these products.
The Federal Emergency Management Agency and the Department of Health and Human Services created a task force to procure and distribute personal protective equipment and medical supplies to states, tribes, and territories. On March 29, FEMA launched Project Air Bridge to reduce the time it takes U.S. medical supply distributors to get supplies from overseas. FEMA pays for the flights, which have reduced shipment times from weeks to days.
SEnate Solutions and PRoposals
The coronavirus pandemic has intensified the broader discussion about whether the U.S. depends too heavily on China for medical devices, pharmaceuticals, and similar critical products. Congress began to address questions about the U.S. supply chain for these medical products in the CARES Act. The law includes provisions to enhance the FDA’s ability to more quickly identify potential shortages and deal with them before they become major challenges to the supply chain. It asks the National Academies of Sciences, Engineering, and Medicine to evaluate the vulnerabilities to U.S. medical supply chain, including any economic impact of increasing domestic manufacturing, and offer recommendations to secure U.S. supply chains. The CARES Act also has provisions to determine the volume of active pharmaceutical ingredients supplied by foreign markets. The FDA already has a registry of all facilities that make drugs and medical devices for sale in the U.S. and tracks the percentage of manufacturers the U.S. relies on in foreign markets, but the amount of the ingredients coming from other countries is not clear.
How the CARES Act Addresses the Medical Supply Chain
Some experts have raised concerns that requiring supply chains to move back to the U.S. right now could lead to further disruption as the U.S. works to source necessary supplies for the COVID-19 pandemic, including increasing the cost of drugs and medical supplies. This could lead to higher health care costs for patients and the government. According to the FDA, labor costs are lower in foreign countries like China and India, allowing 30% to 40% lower costs for U.S.-based companies to manufacture pharmaceutical ingredients.
Republican Senators have offered proposals aimed at making the U.S. less dependent on other countries for critical items such as medical supplies in the longer term.
Senate Small Business Committee Chairman Marco Rubio has sought to increase U.S. small businesses’ domestic production of critical goods. The Small Business Act reauthorization he proposed in 2019 would increase the funding allocations that federal agencies must dedicate to two programs that connect small businesses with federal research and development. They allow the small businesses to commercialize technological innovations that come from these partnerships. The bill also would modernize the SBA’s investment company program, which provides public-private capital for innovation in small businesses.
Senator Josh Hawley’s Medical Supply Chain Security Act would expand the FDA’s authority to require that medical product manufacturers report on the origins of essential drugs and medical devices, along with their component parts. The bill would require medical device manufacturers to tell the FDA of any anticipated shortages or interruptions, which would allow the agency to monitor shortages more accurately and then take action to speed the approval of new devices to market.
Senator Tom Cotton has introduced a bill requiring the FDA to create a registry to track pharmaceuticals and active pharmaceutical ingredients sourced outside the U.S. Beginning in 2024, HHS, Veterans Affairs, the Defense Department, and federally qualified health facilities would be prohibited from purchasing or providing reimbursements for pharmaceuticals with active ingredients from China. The bill calls for increased sourcing transparency by requiring drug manufacturers to list ingredients and their country of origin on pharmaceutical labels. It would provide temporary expensing for manufacturers that expand their production capacity in the U.S., to encourage domestic production.
Senator Marsha Blackburn introduced a bipartisan proposal with Senator Robert Menendez, the Securing America’s Medicine Cabinet Act, that seeks to expand U.S. manufacturing of pharmaceutical ingredients. The bill also would help equip workers for U.S. manufacturing jobs by setting up new centers of excellence that would develop advanced manufacturing for the pharmaceutical industry.
Senator Kelly Loeffler also recently introduced a comprehensive economic recovery plan that includes tax credits, full expensing deductions, and a payroll tax holiday to give U.S. firms incentives to shift their production back to the U.S. The plan seeks to reduce the country’s dependence on imported goods ranging from pharmaceuticals to car parts.
A bipartisan proposal sponsored by Senator Marco Rubio would require DOD to find out how much it relies on foreign entities for drugs and pharmaceutical components, and to make recommendations to reduce dependence. The bill would add additional reporting requirements for drug manufacturers, directing them to tell the FDA the volume of active ingredients used in their pharmaceuticals. It would allow the VA to determine a drug’s source based on where the ingredient was obtained, rather than where the final drug was manufactured.
Next Article Previous Article

